HEPATIC METABOLISM AND LIVER REGENERATION
Martine DAUJAT

Leader
Contact information
Ph : +33 (0) 4 67 33 06 23
Email : martine.daujat@inserm.fr
The group members
DAUJAT-CHAVANIEU Martine (CRHC/Inserm) https://orcid.org/0000-0001-5560-1610
GERBAL-CHALOIN Sabine (CRHC/Inserm) https://orcid.org/0000-0002-2549-7899
BRIOLOTTI Philippe (AI/Inserm)
HERRERO Astrid (MCU-PH/CHU)
BLANC Pierre (PU-PH/CHU)
NAVARRO Francis (PU-PH/CHU)
SOMMIER Lazare (M2 student)
Keywords
Primary human hepatocytes
Detoxication
Metabolism
Nuclear Receptors
Stem cells and liver progenitors
Co-culture
3D Culture
Regeneration
NAFLD/Steatosis
Biotherapy
Know how
In vitro models
Isolation and culture of primary human hepatocytes and other cell types from the liver (sinusoidal, Kupffer cells, liver progenitors…)
Culture and in vitro differentiation of human embryonic stem cells and liver progenitors into hepatocytes
Micro organized co-culture, spheroids, culture under flow
Experimental mouse models
Regeneration and NAFLD/NASH
Phenotypic and functional characterization
Our work is based on an original model of primary cultures of human adult hepatocytes (PHH) and other hepatic cells (liver progenitors, sinusoidal, stellate, Kupffer cells) including mesenchymal cells, which allow investigating several aspects of hepatic physiopathology: liver detoxication functions, steatosis, cholestasis and liver niche. It combines basic and translational research.
Human hepatocytes are isolated by a two-step collagenase perfusion method, either from organ donor livers that are rejected for transplantation or from liver fragments resected for different medical purposes (metastasis, hepatocellular carcinoma or other pathologies). We have established different experimental conditions for monolayer culture, co-culture with other liver cells, 3D organization and dynamic culture to maintain the phenotype and function of the cells for several weeks.
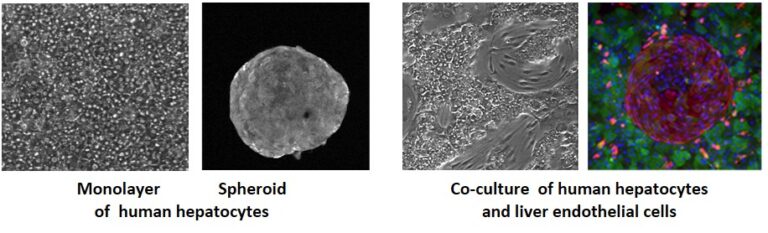
The PHH model is considered as a gold standard for human physiopathological studies.
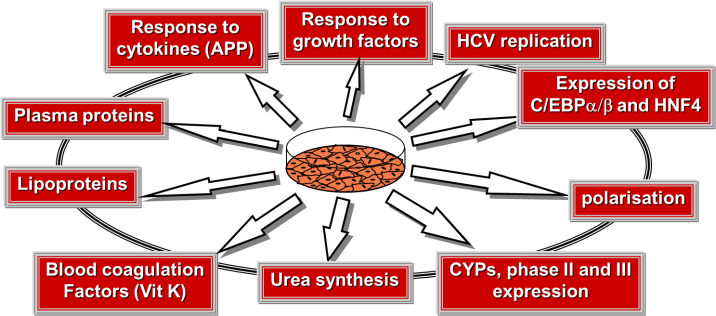
- Detoxification and nuclear receptor cross-talk
The PHH model has been extensively exploited in the lab in the field of xenobiotic and drug metabolism, side effects and toxicity, as well as in the field of drug metabolism enzyme gene regulation including cross talks of the involved nuclear receptors including AhR (the Aryl hydrocarbon Receptor), PXR (Pregnane X Receptor), and CAR (Constitutive Androstane Receptor) with other signaling pathways and their clinical consequence. For instance, a functional interference between the xenoreceptor PXR, the thyroid hormone receptor (TR) and fatty acid synthesis was evidenced, establishing a molecular mechanistic link between xenobiotics and nonalcoholic fatty liver disease (NAFLD) that is frequently observed in patients under chronic treatment with PXR agonists.
- Hepatitis C virus infection
HCV infection is a major cause of chronic liver disease. We demonstrated for the first time that naive PHH were sensitive to HCV infection and permissive to viral genome replication when exposed to sera from chronically infected patients. We identified critical factors leading to efficient PHH infection by HCV sera in vitro and showed that this cellular model provides a useful tool for studying the mechanism of HCV infection, and for determining the potency of drugs towards various HCV strains. Importantly we collected sera from HCV genotype 1 to 6 infected patients and identified a highly infectious genotype 3a strain and established the first robust genotype 3a replicon system and a unique infectious cell system for genotype 3 studies.
- Role of Wnt/β-catenin pathway on hepatocyte metabolic function
In the liver, the lobule organization is complex and is sustained by multiple parameters including blood flow direction, the oxygen and circulating molecules gradients, the extracellular matrix composition, cellular cooperation and signalling pathways activations. These various integrated signals generate a functional zonation of hepatocytes (and other cell types), mainly regulated by Wnt/β-Catenin and APC (Adenomatous polyposis coli) pathways. Most of them are lost when hepatocytes are removed from their natural niche and cultured. We recently showed that the WNT/β-catenin pathway is a transcriptional regulator of CYP2E1, CYP1A2, and aryl hydrocarbon receptor gene expression in primary human hepatocytes, accordingly to their in vivo centro-lobular zonation. Transcriptome analysis of PHH treated with GSK3β inhibitors or invalidated for APC expression revealed that this pathway could be involved also in different aspect of the main hepatocyte functions. Among them, enzymes and proteins involved in bile acid homeostasis, lipid metabolism and cell polarity are deregulated. Concomitantly, bile canalicular structure, functionality and number are affected. We are thus studying the role of WNT/β-catenin signaling on the steatosis/cholestasis balance in human hepatocytes to identify new mechanisms regulating lipid and bile acids homeostasis in human liver and therefore potential targets for the development of new therapeutic approaches.
- Regulation of lipid metabolism by hepatocytes and immune cells
Non-alcoholic fatty liver disease (NAFLD) is a chronic hepatic disorder strongly associated with obesity, type 2 diabetes, and insulin resistance. Worldwide, the prevalence of NAFLD is estimated between 5% and 51%, depending on the study. The spectrum of changes in the liver in NAFLD ranges from simple triglyceride accumulation in hepatocytes (steatosis) to more severe steatosis coupled with inflammation and fibrosis resulting in nonalcoholic steatohepatitis (NASH), which occasionally progresses to cirrhosis and has been associated with the growing prevalence of the hepatocellular-carcinoma. Animal models provide an important research tool and allow many informative mechanistic studies of NASH development. However, due to significant differences in liver metabolism across species, supplementation of these animal data with assays able to assess human-relevant responses is needed.
We recently developed a long term, micropatterned co-culture model of human hepatocytes with stromal cells mimicking hepatic chronic steatosis. The role of the immune system and the ERK5 pathway will be investigated in this model after implementation with an inflammatory signal that prevails during NASH progression. This novel culture platform could be a valuable tool for various in vitro applications, including pharmacological and drug screening studies for possible reversion of steatosis/lipotoxicity (for example metabolic drugs, FXR agonists) and for the prediction of secondary steatosis induced by drugs or testing how endocrine disruptor chemicals may worsen NAFLD.
Liver biotherapy
- Stem cell differentiation to hepatocytes
The scarcity of human liver availability and the inability of human hepatocyte to proliferate in vitro prompted us to develop stem cell-based strategies, using human embryonic stem cells (hES) and adult liver progenitors (LPC). We isolated a heterogeneous epithelial population comprising LPCs from the non-parenchymal fraction of healthy human livers. When cultured in appropriate conditions, the LPC acquire certain functions of the hepatocyte. We have also established a differentiation protocol to direct hES cells towards the hepatocyte lineage. However, as described by others, we face the problem of incomplete differentiation of hES and LPC, suggesting that additional factors are required for terminal maturation.
- Isolation of Hepatocytes in GMP conditions
An alternative to the shortage of organ for liver transplantation is the infusion of isolated hepatocytes to bridge patients to liver transplantation or to spontaneous recovery.
We have adapted our isolation protocol to prepare human hepatocytes in clean GMP conditions. In these conditions, we have succeeded in isolating high-quality hepatocytes from highly steatosic livers of organ donors not used for transplantation, as efficiently as from hepatectomies. We defined conditions for the cold-preservation of human hepatocytes in suspension maintaining their viability and functionality for at least 8h after isolation. The safety and validation of this method of preservation was demonstrated by preclinical transplantation experiments in a mouse model of liver repopulation. This time window could allow for cell transportation, patient preparation and repeated infusions.
- MSC and liver regeneration
The current consensus suggests that the nature of cells to be injected will depend on the liver pathology. The significant contribution of MSC paracrine activity, rather than their ability to differentiate, to the liver reparative process has already been established in a large number of pre-clinical models. We recently showed that a patch of MSC grown on a human amniotic membrane (HAM) improves mice survival and liver regeneration, when applied in direct contact with the remnant liver after extensive hepatectomy. It also decreased the severity of hepatectomy-induced steatosis, suggesting a modulation of lipid metabolism in hepatocytes. These encouraging results pave the way to potential clinical application. We intend now to use MSCs patches to improve regeneration and/or to prevent post-hepatectomy liver failure in our NAFLD/NASH mouse model. Our goal is to better define the immune components of the regenerative liver, which could allow the development of targeted therapeutic strategies to promote hepatic regeneration in patients with liver disease and/or requiring extensive hepatectomy.
In close collaboration with several departments of CHU Saint Eloi hospital, we are currently developing a biotherapy program aiming to transplant adipose derived stem cells in patients with severe liver failure (PHRC in progress).
Bibliographical references
Main Publications (last six years)
-Daujat-Chavanieu M, Kot M. Albumin is a secret factor involved in multidirectional interactions among the serotoninergic, immune and endocrine systems that supervises the mechanism of CYP1A and CYP3A regulation in the liver. Pharmacol Ther. 2020 Jun 23:107616. PMID: 32590025
-Lambert K, Gondeau C, Briolotti P, Scheuermann V, Daujat-Chavanieu M, Aimond F. Biocompatible modified water as a non-pharmaceutical approach to prevent metabolic syndrome features in obesogenic diet-fed mice. Food Chem Toxicol. 2020 May 6;141:111403. PMID: 32387306
-Creusot N, Gassiot M, Alaterre E, Chiavarina B, Grimaldi M, Boulahtouf A, Toporova L, Gerbal-Chaloin S, Daujat-Chavanieu M, Matheux A, Rahmani R, Gongora C, Evrard A, Pourquier P, Balaguer P. Cells. 2020 Jul 8;9(7):1641. PMID: 32650447
-Daujat-Chavanieu M, Gerbal-Chaloin S. Regulation of CAR and PXR Expression in Health and Disease. Cells. 2020 Oct 31;9(11):2395. Review. PMID: 33142929
-Ul Haq Khan A, Allende-Vega N, Gitenay D, Garaude J, Dang N, Belkhala S, Gerbal-Chaloin S, Gondeau C, Daujat-Chavanieu M, Delettre C, Orecchioni S, Talarico G, Bertolini F, Anel A, Cuezva JM, Enriquez JA, Cartron G, Lecellier CH, Hernandez J, Villalba M. Mitochondrial Complex I activity signals antioxidant response through ERK5. 2018. Scientific Reports May 9;8(1):7420.
-Abderrahmani A, Yengo L, Caiazzo R, Canouil M, Cauchi S, Raverdy V, Plaisance V, Pawlowski V, Lobbens S, Maillet J, Rolland L, Boutry R, Queniat G, Kwapich M, Tenenbaum M, Bricambert J, Saussenthaler S, Anthony E, Jha P, Derop J, Sand O, Rabearivelo I, Leloire A, Pigeyre M, Daujat-Chavanieu M, Gerbal-Chaloin S, Dayeh T, Lassailly G, Mathurin P, Staels B, Auwerx J, Schurmann A, Postic C, Schafmayer C, Hampe J, Bonnefond A, Pattou F, Froguel P. Increased Hepatic PDGF-AA Signaling Mediates Liver Insulin Resistance in Obesity-Associated Type 2 Diabetes. 2018. Diabetes 67(7): 1310-1321
-Da-Silva F, Boulenc X, Vermet H, Compigne P, Gerbal-Chaloin S, Daujat-Chavanieu M, Klieber S, Poulin P. Improving Prediction of Metabolic Clearance Using Quantitative Extrapolation of Results Obtained From Human Hepatic Micropatterned Cocultures Model and by Considering the Impact of Albumin Binding. 2018. J Pharm Sci 107(7): 1957-1972
-de Boussac H, Gondeau C, Briolotti P, Duret C, Treindl F, Romer M, Fabre JM, Herrero A, Ramos J, Maurel P, Templin M, Gerbal-Chaloin S, Daujat-Chavanieu M. Epidermal Growth Factor Represses Constitutive Androstane Receptor Expression in Primary Human Hepatocytes and Favors Regulation by Pregnane X Receptor. 2018. Drug Metab Dispos 46(3): 223-236
-Despeyroux A, Duret C, Gondeau C, Perez-Gracia E, Chuttoo L, de Boussac H, Briolotti P, Bony C, Noel D, Jorgensen C, Larrey D, Daujat-Chavanieu M*, Herrero A*. Mesenchymal stem cells seeded on a human amniotic membrane improve liver regeneration and mouse survival after extended hepatectomy. 2018. J Tissue Eng Regen Med 12(4): 1062-1073 * Equal contribution.
-Kot M and Daujat-Chavanieu M. Altered cytokine profile under control of the serotonergic system determines the regulation of CYP2C11 and CYP3A isoforms. 2018. Food Chem Toxicol 116(Pt B): 369-378
-Belkahla S, Ul Haq Khan A, Gitenay D, Alexia C, Gondeau C, Vo DN, Orecchioni S, Talarico G, Bertolini F, Cartron G, Hernandez J, Daujat-Chavanieu M, Allende-Vega N, Villalba Gonzalez M. Changes in Metabolism Controls expression of ABC Transporters Through ERK5 and Depending on p53 status. 2018. Oncotarget Dec 14;9(1):1114-1129.
-Ul Haq Khan A, Allende-Vega N, Gitenay D, Gerbal-Chaloin S, Gondeau C, Vo DN, Belkhala S, Orecchioni S, Talarico G, Bertolini F, Bozic M, Valdivielso JM, Bejjani F, Jariel I, Lopez-Mejia IC, Fajas L, Lecellier CH, Hernandez J, Daujat M, Villalba M. The PDK1 Inhibitor Dichloroacetate Controls Cholesterol Homeostasis Through the ERK5/MEF2 Pathway. 2017. Scientific Reports Sep 6;7(1):10654.
-Cabantous S, Hou X, Louis L, He HB, Mariani O, Sastre X, Daujat-Chavanieu M, Li Y, Dessein A. Evidence for an important role of host microRNAs in regulating hepatic fibrosis in humans infected with Schistosoma japonicum. 2017. Int J Parasitol 47(13): 823-830
-Herrero A, Prigent J, DLombard C, Rosseels V, Daujat-Chavanieu M, Breekpot K, Najimi M, Deblandre G, Sokal EM. Adult-Derived Human Liver Stem/Progenitor Cells Infused 3 Days Postsurgery Improve Liver Regeneration in a Mouse Model of Extended Hepatectomy. 2017. Cell Transplant 26(2): 351-364
-Iroz A, Montagner A, Benhamed F, Levavasseur F, Polizzi A, Anthony E, Regnier M, Fouche E, Lukowicz C, Cauzac M, Tournier E, Do-Cruzeiro M, Daujat-Chavanieu M, Gerbal-Chaloin S, Fauveau V, Marmier S, Burnol AF, Guilmeau S, Lippi Y, Girard J, Wahli W, Dentin R, Guillou H, Postic C. A Specific ChREBP and PPARalpha Cross-Talk Is Required for the Glucose-Mediated FGF21 Response. 2017. Cell Rep 21(2): 403-416
-Rasmussen MK, Daujat-Chavanieu M, Gerbal-Chaloin S. Activation of the aryl hydrocarbon receptor decreases rifampicin-induced CYP3A4 expression in primary human hepatocytes and HepaRG. 2017. Toxicol Lett 277: 1-8
-Nzigou Mombo B, Gerbal-Chaloin S, Bokus A, Daujat-Chavanieu M, Jorgensen C, Hugnot JP, Vignais, ML. MitoCeption: Transferring Isolated Human MSC Mitochondria to Glioblastoma Stem Cells. 2017. J Vis Exp(120)
-Ul Haq Khan A, Rathore MG … Lecellier CH, Villalba M. Human leukemic cells performing oxidative phosphorylation (OXPHOS) generate an antioxidant response independently of reactive oxygen species (ROS) generation. 2016. EBioMedicine 3 pp 43-53. Comments on: Keep Harm at bay: Oxidative Phosphorylation Induces Nrf2-Driven Antioxidant Response via ERK5/MEF2/miR-23a Signaling to Keap-1. Danilenko M, Studzinski GP. EBioMedicine. 2016 Jan 11;3:4-5.
-Kot M, Daujat-Chavanieu M. The impact of serotonergic system dysfunction on the regulation of P4501A isoforms during liver insufficiency and consequences for thyroid hormone homeostasis. 2016. Food Chem Toxicol 97: 70-81
-Rasmussen MK, Balaguer P… Daujat-Chavanieu M, Gerbal-Chaloin S. Skatole (3-Methylindole) Is a Partial Aryl Hydrocarbon Receptor Agonist and Induces CYP1A1/2 and CYP1B1 Expression in Primary Human Hepatocytes. 2016. PLoS One 11(5): e0154629
-Romano A, Hou X… Li Y, Dessein A. Correction: FOXP3+ Regulatory T Cells in Hepatic Fibrosis and Splenomegaly Caused by Schistosoma japonicum: The Spleen May Be a Major Source of Tregs in Subjects with Splenomegaly. 2016. PLoS Negl Trop Dis 10(2): e0004454
-Azzag K, Chelin Y, Rousset F, Le Goff E, Martinand-Mari C, Martinez AM, Maurin B, Daujat-Chavanieu M, Godefroy N, Averseng J, Mangeat P, Baghdiguian S. The non-proliferative nature of ascidian folliculogenesis as a model of highly ordered cellular topology distinct from proliferative epithelia. 2015. PLoS One 10(5): e0126341
-Briolotti P, Chaloin L, Balaguer P, Da Silva F, Tomankova V, Pascussi JM, Duret C, Fabre JM, Ramos J, Klieber S, Maurel P, Daujat-Chavanieu M, Gerbal-Chaloin S. Analysis of Glycogen Synthase Kinase Inhibitors That Regulate Cytochrome P450 Expression in Primary Human Hepatocytes by Activation of beta-Catenin, Aryl Hydrocarbon Receptor and Pregnane X Receptor Signaling. 2015. Toxicol Sci 148(1): 261-275
-Delfosse V, Dendele B, Huet T, Grimaldi M, Boulahtouf A, Gerbal-Chaloin S, Beucher B, Roecklin D, Muller C, Rahmani R, Cavailles V, Daujat-Chavanieu M, Vivat V, Pascussi JM, Balaguer P, Bourguet W. Synergistic activation of human pregnane X receptor by binary cocktails of pharmaceutical and environmental compounds. 2015. Nat Commun 6: 8089
-Duret C, Moreno D, Balasiddaiah A, Roux S, Briolotti P, Raulet E, Herrero A, Ramet H, Biron-Andreani C, Gerbal-Chaloin S, Ramos J, Navarro F, Hardwigsen J, Maurel P, Aldabe R, Daujat-Chavanieu M. Cold Preservation of Human Adult Hepatocytes for Liver Cell Therapy. 2015. Cell Transplant 24(12): 2541-2555
-Marmier S, Dentin R, Daujat-Chavanieu M, Guillou H, Bertrand-Michel J, Gerbal-Chaloin S, Girard J, Lotersztajn S, Postic C. Novel role for carbohydrate responsive element binding protein in the control of ethanol metabolism and susceptibility to binge drinking. 2015. Hepatology 62(4): 1086-1100
-Gerbal-Chaloin S, Dumé AS, Briolotti P, Klieber S, Raulet E, Duret C, Fabre JM, Ramos J, Maurel P, Daujat-Chavanieu M. The WNT/beta-catenin pathway is a transcriptional regulator of CYP2E1, CYP1A2, and aryl hydrocarbon receptor gene expression in primary human hepatocytes. 2014a. Mol Pharmacol 86(6): 624-634
-Gondeau C, Briolotti P, Razafy F, Duret C, Rubbo PA, Helle F, Reme T, Ripault MP, Ducos J, Fabre JM, Ramos J, Pecheur EI, Larrey D, Maurel P, Daujat-Chavanieu M. In vitro infection of primary human hepatocytes by HCV-positive sera: insights on a highly relevant model. 2014. Gut 63(9): 1490-1500
Book chapters
-Herrero A, Gerbal-Chaloin S and Daujat-Chavanieu M. Bioinspired Liver Tissue Engineering. Book chapter in Handbook of Biomimetics and Bioinspiration. 2014. Eds Jabbari E, Khademhosseini A, Lee LP, Kim DH, Ghaemmaghami A. World Scientific Publishing Co, Pte, Ltd.

