
APPARAILLY Florence
(DR1 / INSERM)
COURTIES Gabriel
(CRCN / INSERM)
BORTOLOTTI Olivier
(CR / CDD INSERM)
TAFFONI Clara
(Post-Doc, CDD IHU)
PONSOLLES Clara
(IE, INSERM)
GRAVERON Guilhem
(PhD, ED CBS2, Univ Montpellier)
ALVARES Natalia
(Tech, CDD)
PARET Chloé
(Master 2)
Our laboratory is dedicated to deciphering the complex roles of myeloid cells in chronic inflammatory and fibrotic diseases. We believe that by understanding how these cells function and interact within diseased tissues, we can design transformative therapies to restore tissue balance and resolve inflammation. Our research is organized around three interconnected axes:
1. Mapping the Cellular Ecosystems of Rheumatic Disease
How do immune cells orchestrate the chronic inflammation and tissue damage that characterize diseases like Rheumatoid Arthritis, Osteoarthritis, and Systemic Sclerosis?
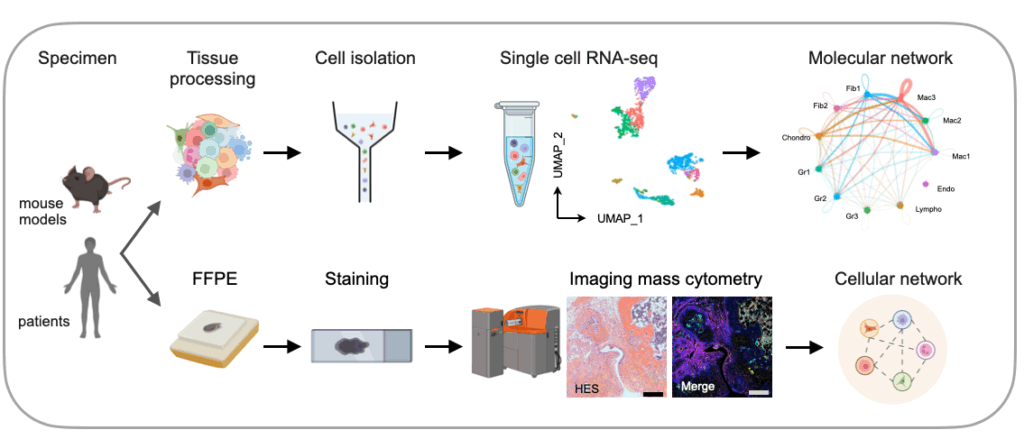
Chronic inflammation is driven by the dysregulated dialogue between cellular players within affected tissues. Myeloid cells—particularly the distinct populations of tissue-resident (resMac) and infiltrating monocyte-derived (moMac) macrophages—are central hubs in this process. They engage in constant communication with other immune and stromal niche cells, either maintaining tissue health or driving a vicious cycle of inflammation and fibrosis (Trends Immunol. 2021).
By integrating single-cell RNA sequencing, imaging mass cytometry, and spectral flow cytometry with genetic fate-mapping mouse models, we are mapping these intricate cellular interactions in health and disease (Arthritis Rheumatol. 2025, Ann Rheum Dis. 2025). Our ultimate goal is to unravel these specific cellular crosstalks and pathogenic networks to discover novel biomarkers for patient stratification and pinpoint precise therapeutic targets for restoring tissue homeostasis and resolving disease.
2. Therapeutic Reprogramming of Myeloid Cells
Instead of broadly suppressing the immune system, can we precisely re-educate myeloid cells, instructing them to resolve inflammation
and promote tissue repair?
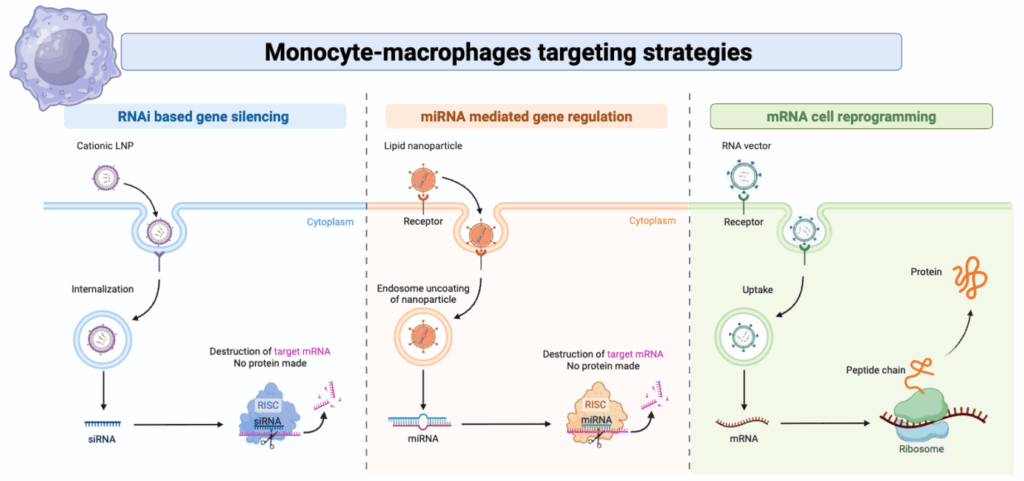
Current treatments for autoimmune diseases often rely on broad immunosuppression, leaving patients vulnerable to infections. Our vision is to develop a new generation of therapies that can selectively reprogram pro-inflammatory myeloid cells in vivo, turning them from drivers of disease into agents of resolution. To achieve this, we are harnessing the power of RNA-based nanomedicine (Blood. 2010, Ann Rheum Dis. 2013, Theranostics. 2018).
This innovative strategy involves two key components: the “message” and the “envelope.” The message consists of synthetic, modified messenger RNA (mRNA) and the envelope is a custom-designed nanovector designed to protect the mRNA and deliver it specifically to
target macrophage subsets in diseased tissues. By validating this platform from in vitro assays to preclinical models of arthritis and sclerosis, we aim to establish a powerful new therapeutic modality capable of restoring tissue homeostasis from within.
3. The microRNA Rheostat: Fine-Tuning Myeloid Cell Fate & Function
What are the molecular switches that govern a myeloid cell’s identity and behavior, and how are they dysregulated in disease?
Beyond the genetic code, a cell’s function is dictated by layers of regulatory control. Our team has a long-standing expertise in microRNAs (miRNAs), small non-coding RNA molecules that act as master rheostats, capable of fine-tuning entire gene networks (Nat Rev Rheumatol. 2016, Front Immunol. 2019). In myeloid cells, miRNAs are critical for controlling fundamental processes like differentiation, activation, and polarization, determining whether a macrophage becomes inflammatory or pro-resolving (Blood. 2016).
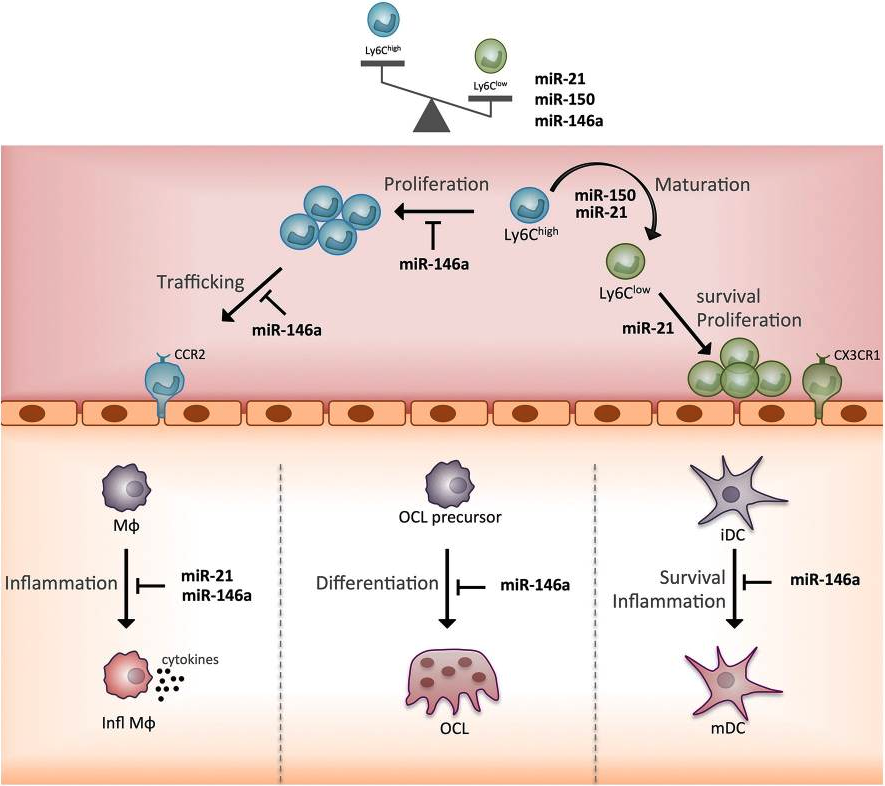
We investigate how specific miRNAs and their targets control monocyte and macrophage functions, both in health and in diseases. By dissecting these intricate regulatory circuits, we aim to understand the fundamental rules governing myeloid cell identity. This knowledge provides a source of novel diagnostic markers and uncovers new pathways that can be therapeutically targeted.
 Lauréat i-Lab 2025 !
Lauréat i-Lab 2025 !
Episode 1 de la websérie de la Fondation Arthritis “Paroles de chercheurs”