

PELEGRIN Mireia (DR2 / CNRS)
NARANJO-GOMEZ Mar (CRCN / INSERM)
ABBA-MOUSSA Daouda (IR, CDD / CHU Montpellier)
SOUCHARD Manon (IE, CDD / UM)
BRU Rémi (PhD-Student / UM)
PARIS Océane (post-doc / INSERM)
BERETTA Maxime (post-doc / INSERM)
LABAYE Quiterie (PhD-Student / UM)
MILAZZO Louis-Antoine (CIFRE PhD-Student UM / Cilcare)
Immunotherapy
Monoclonal antibodies
IFN-I
Immunomodulation
Innate and adaptive immunity
Neutrophils
Immune complexes
Cell therapy
Autoimmunity
Rheumatoid Arthritis
Characterization of innate and adaptive immune responses in humans and mice
Structure-function relationship of antibodies
Interferon systems in humans and mice: from ligands to signaling
Experimental models of viral infections and cancer
Complex cellular models
Organoids


Quiterie Labaye (PhD student) and Mar Naranjo (CRCN-Inserm) at the
38e Congrès de la Société Française de Rhumatologie :
14-16 December 2025, Porte de Versailles
https://congres.larhumatologie.fr/
Summary:
Our research project aims to develop innovative immunotherapies for dys-immune diseases. Our work has pioneered the proof-of-concept that monoclonal antibodies (mAb) are immunomodulatory agents that can induce the development of a long-term protective immune response (“vaccinal effects”). On the other hand, antibodies against autologous structures (auto-antibodies) can develop and cause different forms of tissue damage causing autoimmune diseases. It is now clear that the impact of antibodies (therapeutic mAb or auto-antibodies) on the induction of the immune response involves different mechanisms among which Fc-Fc receptor (FcR) interactions and type I interferons (IFN-I) are key actors. A major challenge is therefore to identify these mechanisms (common or specific) involved in the induction of the antibody-mediated immune response. This would enable us to control them in order to promote them in the context of immunotherapies for infectious or cancerous diseases or, on the other hand, to slow them down in the context of autoimmune diseases by developing innovative immunotherapies. Thus, using in vitro, ex vivo and in vivo approaches, our work aims to study the role of mAbs/autoantibodies in the recruitment and activation of immune system cells. Different mouse models of pathologies (notably, viral infections and rheumatoid arthritis), complex cellular models (including organoids) and patient cells are used to address this issue. We aim to identify new molecular and cellular targets as a basis for the development of new antibody-based and cell-based therapeutic interventions for the treatment of viral and autoimmune diseases, with a particular focus on rheumatoid arthritis, HIV-1- and SARS-CoV2 infection.
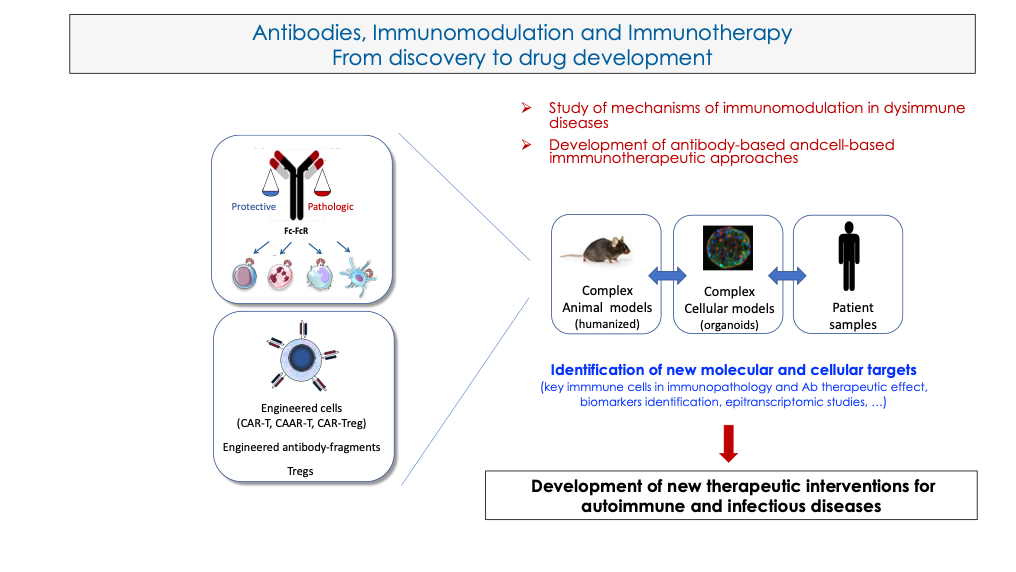
Main achievements:
1. Induction of vaccinal effects by antiviral mAbs:
a. Identification and conceptualization of mechanisms involved in the induction of vaccinal effects by antiviral antibodies
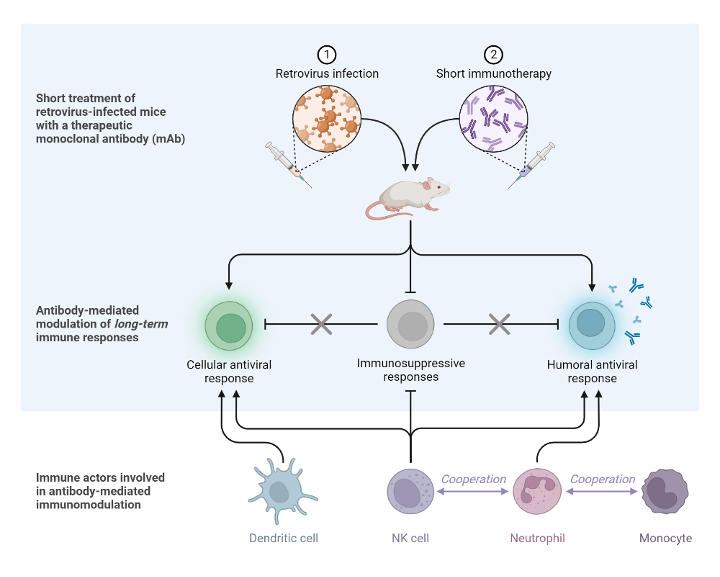
Using a mouse model of retroviral infection, we have identified and conceptualized the mechanisms involved in the induction of vaccinal effects by antiviral antibodies. Our finding highlights a key role of Fc-FcR interactions in the induction of protective humoral and cellular immune responses. They also highlight the role of different FcR-expressing cells, such as dendritic cells, neutrophils and NK cells, as well as the cooperation among these different cell types. The team is a leader opinion in the field and has been invited to write several reviews.
https://www.ncbi.nlm.nih.gov/pubmed/20548955
https://www.ncbi.nlm.nih.gov/pubmed/23264590
https://www.ncbi.nlm.nih.gov/pubmed/29720574
https://pubmed.ncbi.nlm.nih.gov/33858301
https://pubmed.ncbi.nlm.nih.gov/33567792/
https://www.ncbi.nlm.nih.gov/pubmed/26433697 https://www.ncbi.nlm.nih.gov/pubmed/27530750 Mechanisms involved in the induction of vaccinal effects.
Naranjo-Gomez et al, 2023, doi: 10.1097/COH.0000000000000797
Created by “M.Pelegrin, M. Naranjo-Gomez and S. Marsile-Medun” using BioRender.com (2023).
b. Therapeutic approaches against West Nile Virus infection
As pioneers in the field of vaccinal effects induced by antiviral antibodies and the modulation of antiviral immune responses, our team is member of the European Consortium “LWNVIVAT” (Limiting West Nile Virus impact by novel vaccines and therapeutics approaches). As the number of WNV infection cases is growing and until now, no prophylaxis or treatment exist, this consortium aims at evaluating the immunogenicity of vaccine prototypes (recombinant proteins and VLPs) and identify an adjuvant system for the induction of a long-lasting antibody responses in a mouse model of WNV infection. Moreover, we will assess candidate vaccines and antibodies in prophylactic and therapeutic approaches. Mireia Pelegrin (WP leader) and Mar Naranjo-Gomez will address the study of the adjuvant effects of anti-WNV antibodies developed in the frame of this consortium.
More information at: https://lwnvivat.eu

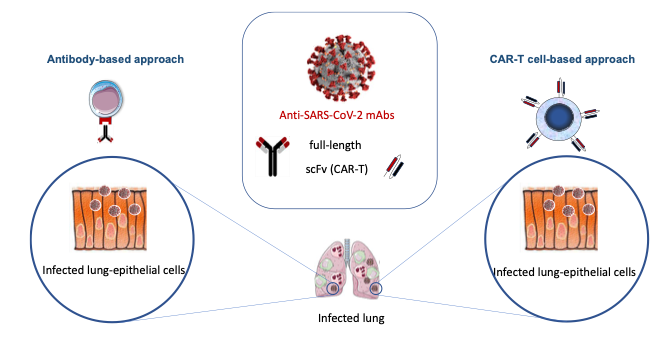
2. Development of antibody-based and cell-based therapeutic approaches against SARS-CoV2 infection
Our team has been a member of the European Consortium “ACT4COVID” which aimed at developing a multi-center research project for a better understanding of how the immune system react in response to SARS-CoV-2 infection, and for the development of new treatments to be proposed according to the host’s cellular response to COVID-191. The project has been supported by a 5 million euros investment through an agreement with the company Cellnex. The consortium included six leading immunotherapy research teams in Europe.
Our team (Mireia Pelegrin/Mar-Naranjo-Gomez WP leaders) has been actively involved in this project Capitalizing on our expertise in antiviral antibodies and immunotherapies. We discovered a pan anti-SARS-CoV2 mAb (C10 mAb) with efficient infected killing capacity for novel antibody-based and cell-based therapeutic approaches. Notably, in collaboration with the team of Manel Juan at the Hospital clinic de Barcelona, we generated CAR-T cells derived from C10, capable of significantly reducing the viral load in vitro. Our work reveals a pan-SARS-CoV-2 mAb effective in targeting infected cells and demonstrates the proof-of-concept for the potential application of CAR-T cell therapy in combating SARS CoV-2 infections.
1 A consortium of European hospitals led by Clínic IDIBAPS is launching a cellular immunotherapy project to tackle COVID-19 with the support of Cellnex Telecom. 2020.
Supported by:

3. Comprehensive analysis of neutrophil immunomodulatory properties and FcR dynamics in health and HIV-1 infection and therapy
We have investigated the immunomodulatory properties of human neutrophils in the context of HIV-1 infection and therapy. We characterized the functional activation and the modulation of Fcγ receptor (FcγR) expression on neutrophils isolated from healthy donors (HD) or people living with HIV-1 (PLWH) upon stimulation with virions, free or in the form of ICs made with broadly neutralizing antibodies (bNAbs). Additionally, we investigated the heterogeneity of granulocyte populations and the potential differences in phenotype and immunomodulatory capacity between LDG and normal density granulocytes (NDG) in people living with HIV-1 (PLWH). Our work sheds light on the properties of LDG in PLWH and provided an extensive characterization of this granulocyte subset in which Fc receptors are key discriminatory markers.
Supported by:

4. Development of innovative tools that allow the targeting of IFN agonists and antagonists in a specific cell type
In collaboration with the team of Jan Tavernier (VIB, Ghent, Belgium), the team (G. Uze, former member) developed innovative tools to target IFN-I signaling in cell-specific way. IFN-I agonistic antibody-derived molecules specific for DC showed improvement of antitumoral immune responses. IFN-antagonistic antibody-derived molecules are currently been studied in the context of oncolytic immunotherapy of thoracic cancers to improve tumor cell targeting. This is currently being studied in collaboration with Jean-François Fonteneau (CRCI2NA, Nantes) in the frame of a INCa financial support (PLBIO).
Supported by:

5. Understanding the pathophysiology of autoimmune diseases in order to develop innovative targeted immunotherapies
Rheumatoid arthritis (RA) is a debilitating autoimmune disease that affects globally millions of people. It is a heterogeneous autoimmune disease characterized by persistent joint inflammation. This inflammation causes stiffness, pain and swelling, and may eventually lead to joint damages due to cartilage and bone destruction.
Control of inflammation is critically important in the treatment of RA because there is a window of opportunity between the onset of inflammation and the onset of structural joint damage. RA is characterized by production of autoantibodies (auto-Abs) against various autoantigens of the mammalian body, in particular the presence of anti-citrullinated anti-protein Abs (ACPA). Those auto-Abs are thought to contribute to the autoimmune disease pathogenesis, either directly by engagement of their target autoantigens or by triggering a non-specific inflammatory reaction. This inflammation and concomitant tissue damage caused by the infiltrating inflammatory cells play a critical role in the induction and perpetuation of the disease including neutrophils (PMN) (among others).
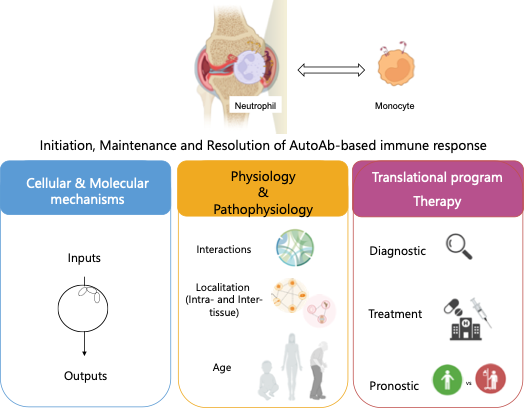
a- Modeling neutrophils’ nole in initiating and perpetuating chronic inflammation using multi-omics approaches
Antibodies recognize targets via the Fab fragment, forming immune complexes (ICs) that trigger inflammation in autoimmune diseases through FcγR binding on immune cells. Understanding auto-Ab mechanisms is crucial for developing counterstrategies. Animal models, particularly the K/BxN serum-transfer arthritis (STA) model, help study auto-Ab-induced pathology. Using STA, we observed a rapid influx of PMNs into inflamed joints at arthritis onset, coinciding with peak symptoms. Our analysis of arthritic joint samples highlights PMNs’ key inflammatory role and their dynamic immunomodulatory functions through secretome and interactome changes. With the support of ANR.
Supported by:



More information at: https://irmb-montpellier.fr/wp-content/uploads/2022/11/livret-IHU-br-1.pdf
b- To transfer basic research to clinical applications
From the clinical, immunological and molecular perspectives, RA is a heterogeneous disease and treatments effective for one patient frequently do not work for another. Due to individual differences, the response rate to this first line treatment currently lies at an inadequate 60-70%. Additionally, despite the success of targeted therapies in the treatment of RA, the lack of predictive biomarkers drives a ‘trial and error’ approach to treatment allocation, leading to variable and/or unsatisfactory responses. Thus, there are still unmet needs to achieve more accurate diagnostics and more effective therapies. Our translational Research Program focused on antibody-mediated responses that engage multiple immune cells, including neutrophils and monocytes, at various levels of interaction. We will plan to acquire high dimensional cellular and molecular relevant data on the integrative analyses of neutrophil biology (NeutroARTherapy, NCT05802719).
Supported by:

6. Generation of Lymphoid Organoids (LOs): a complex cellular model for studying the efficacy of innovative therapies
We are developing LOs from blood-derived cells in collaboration with the company CILCARE. This approach enables the creation of models based on patient-specific cells, thereby reflecting the immunological characteristics of these diseases. This 3D ex vivo system will provide a platform to study immune mechanisms, B-T cell interactions, and B cell responses in diverse pathological contexts, such as vaccine approaches or autoimmune diseases.
Supported by:
